Abstract
Background
Early diagnosis and treatment interventions of transplant-related adverse events (TRAE) such as sepsis, fungal, bacterial and viral infection, graft-versus-host disease (GvHD), veno-occlusive disease (VOD), and graft rejection is crucial for the patient outcome. The predictive value of a multitude of serum biomarkers their specific patterns in occurring TRAE have been widely investigated. Procalcitonin (PCT) is a precursor molecule of the hormone calcitonin that regulates calcium homeostasis and is elevated for example during systemic infection, thermal injury or septic shock. A discriminative value of PCT during adverse events after HSCT has been described for adult patients. There is currently no data available concerning PCT in the context of TRAE in children.
Material and Methods
In this single-center retrospective analysis, serum PCT and CRP levels of pediatric patients who underwent allogeneic HSCT for the treatment of hemato-oncological malignancies and non-malignancies were analyzed and correlated to TRAE (graft rejection, VOD, GvHD, sepsis, and viral or fungal infection). Primary objective of this work was to evaluate a predictive and discriminative value of serum PCT and CRP in the early identification of transplant-related complications in these patients. The observation period included the time of measurement directly before start of conditioning, until the day of clinical discharge. Serum PCT and CRP values were analyzed daily during the occurrence of fever or at least 3 times per week.
Results
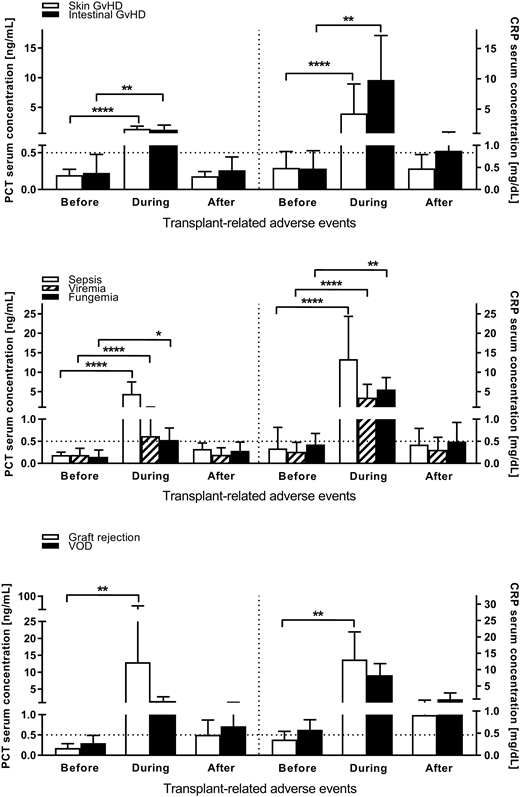
The patient group consisted of 124 male and 87 female pediatric patients (n=211) with a median age of 9.0 years (range 0.5-17.8 years) undergoing HSCT for hemato-oncological malignancies and inborn errors of metabolism. Patients received grafts from mismatched unrelated donors (n=66), matched unrelated donors (n=118) or matched family donors (n=27). The patients underwent HSCT for the treatment of acute leukemia (AML, ALL, AML relapse and ALL relapse; 50.7% of the patients), myelodysplastic syndromes (5.2%), anemia (severe aplastic anemia, beta thalassemia, sickle cell anemia; 16.1%), or solid tumors (28.0%). The median observation period was 76 days (range 31-245 days). Graft rejection occurred in 9 patients (4.3%), VOD in 4 patients (1.9%), acute skin GvHD grade II-IV in 31 patients (14.7%), acute intestinal GvHD grade II-IV in 10 patients (4.7%), sepsis in 24 patients (11.4%), viremia in 22 patients (10.4%), and fungemia in 7 patients (3.3%).
PCT values were significantly and relevantly elevated (>0.5 ng/mL) during graft rejection (median 1.43 ng/mL, range 1.04-5.46 ng/mL), skin GvHD (median 0.90 ng/mL, range 0.02-4.34 ng/mL), intestinal GvHD (median 1.01 ng/mL, range 0.30-3.05 ng/mL), and sepsis (median 3.09 ng/mL, range 0.57-37.32 ng/mL). CRP values were significantly and relevantly elevated (>0.5 mg/dL) during graft rejection (median 10.34 mg/dL, range 5.45-30.19 mg/dL), skin GvHD (median 1.85 mg/dL, range 0.04-21.63 mg/dL), intestinal GvHD (median 8.73 mg/dL, range 1.05-24.2 mg/dL), sepsis (median 10.26 mg/dL, range 2.67-49.47 mg/dL), viremia (median 1.80 mg/dL, range 0.06-14.04 mg/dL), and fungemia (median 5.10 mg/dL, range 2.30-11.60 mg/dL). A simultaneous significant and relevant elevation of both PCT and CRP values was observed during graft rejection, skin and intestinal GvHD, and sepsis (Fig. 1).
Conclusions
Especially during acute skin and intestinal GvHD, PCT and CRP values strongly and significantly increased beyond the upper normal limits. Simultaneous elevations of PCT and CRP occurred during graft rejection, skin and intestinal GvHD, and sepsis. Taken together, laboratory-chemical analyses of serum PCT in combination with CRP could be a useful discriminative biomarker in the detection of TRAE. Larger, prospective trials are needed, to evaluate these findings.
Figure
Fig. 1. PCT and CRP serum concentrations during acute GvHD, graft rejection, VOD, sepsis, viremia and fungemia. Dotted horizontal lines indicate normal PCT and CRP values (PCT: 0.5 ng/mL | CRP: 0.5 mg/dL). Symbols indicate *: p<0.05; **:p<0.01; ***: p<0.001; ****: p<0.0001; tested by Wilcoxon matched-pairs signed rank test.
Acknowledgments
This work was supported by the Stefan-Morsch-Stiftung, Birkenfeld, Germany and the Bettina-Bräu-Stiftung, Fürstenfeldbruck, Germany.
Lang:Miltenyi Biotec: Patents & Royalties, Research Funding. Handgretinger:Miltenyi Biotec: Patents & Royalties: Co-patent holder of TcR alpha/beta depletion technologies, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal